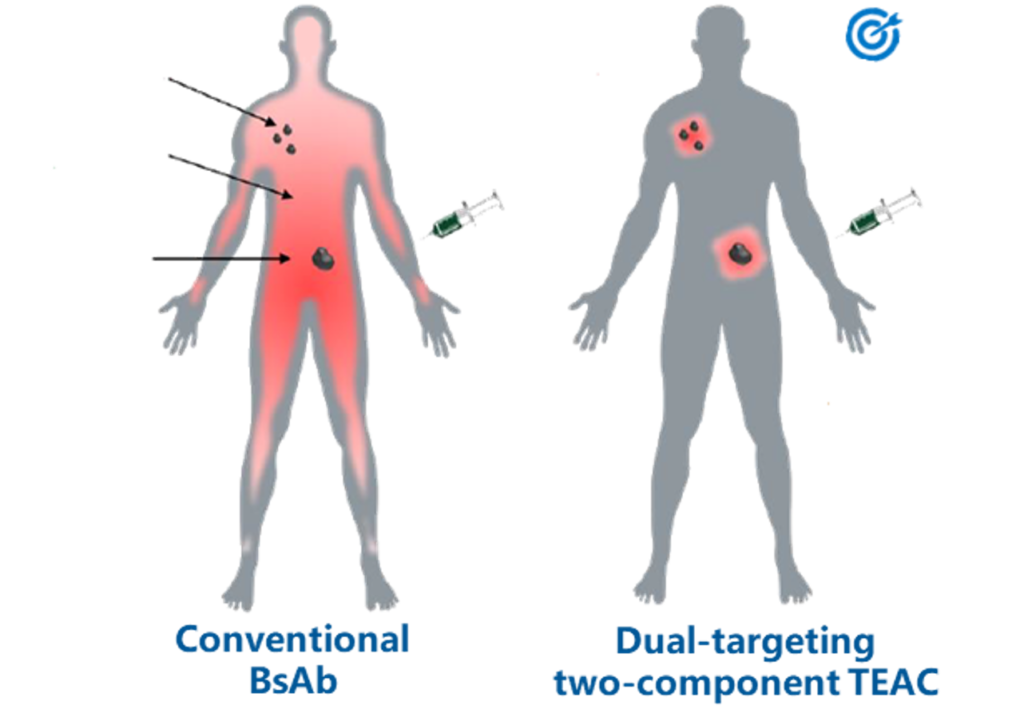
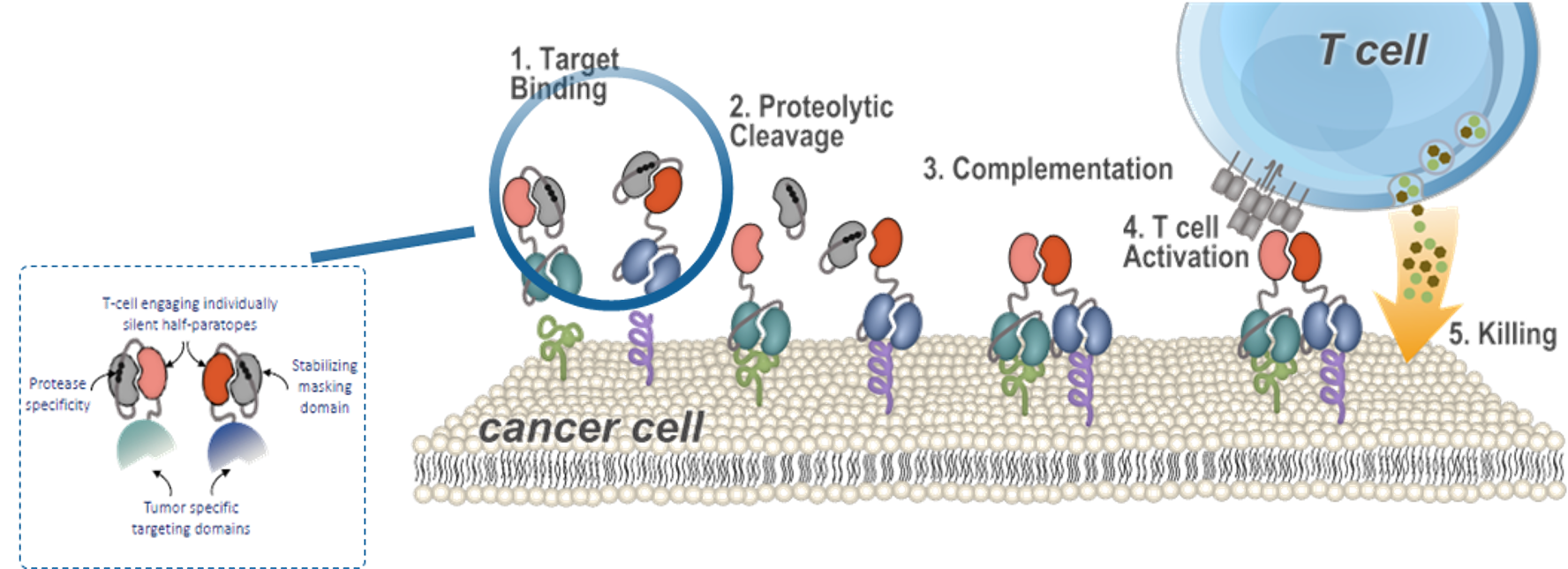
Our high throughput capabilities provides TopAlliance with a foundation for our R&D of innovative monoclonal antibodies and functional screening in vitro and in vivo. We have developed a suite of unique cell lines expressing our desired drug targets along with proprietary assays, humanization capabilities and model systems to accelerate our understanding of target biology, function and mechanism of action.
Our systematic research process enables us to rapidly identify our drug candidate of interest to advance towards clinical development.