- Indications: Cancer (FDA breakthrough designation)
- Clinical trial information: Safety, Tolerability and Pharmacokinetics of an Anti-PD-1 Monoclonal Antibody in Subjects With Advanced Malignancies
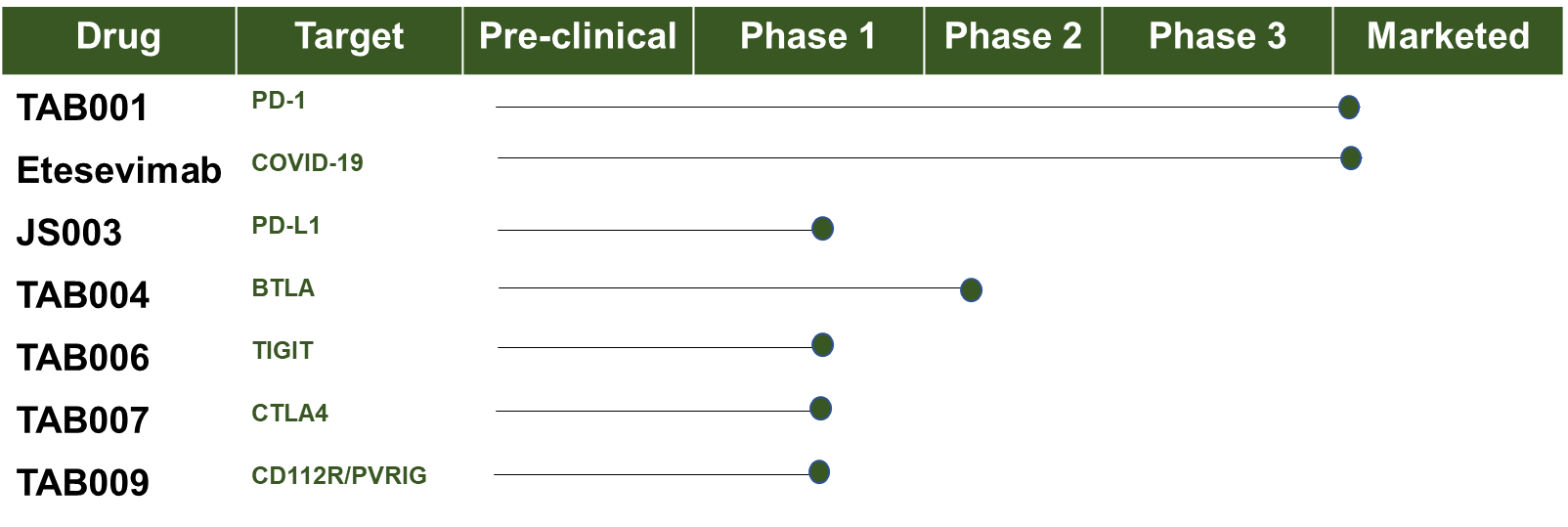
Clinical Trial Information
- Indications: COVID-19
- Clinical trial information: A Study of LY3819253 (LY-CoV555) and LY3832479 (LY-CoV016) in Participants With Mild to Moderate COVID-19 Illness (BLAZE-1)
- Indications: Cancer (Solid tumors)
- Clinical trial information: Safety, Tolerability and Pharmacokinetics of an Anti-PD-1 Monoclonal Antibody in Subjects With Advanced Malignancies
