COVID-19 has devastated societies worldwide. While vaccines are making tremendous progress, patients suffering from COVID-19 complications require innovative and effective therapeutic solutions. Together with our strategic partner, Eli Lilly, we are advancing antibody therapy with a unique mechanism of action

Minimal Adverse Events with Combination
- Monotherapy and combination therapies both well tolerated; no significant concerns
- No clinically meaningful differences in TEAEs were observed across treatment groups
- Study-specific clinical events related to COVID-19 reported separately and not as Adverse Events (per protocol)
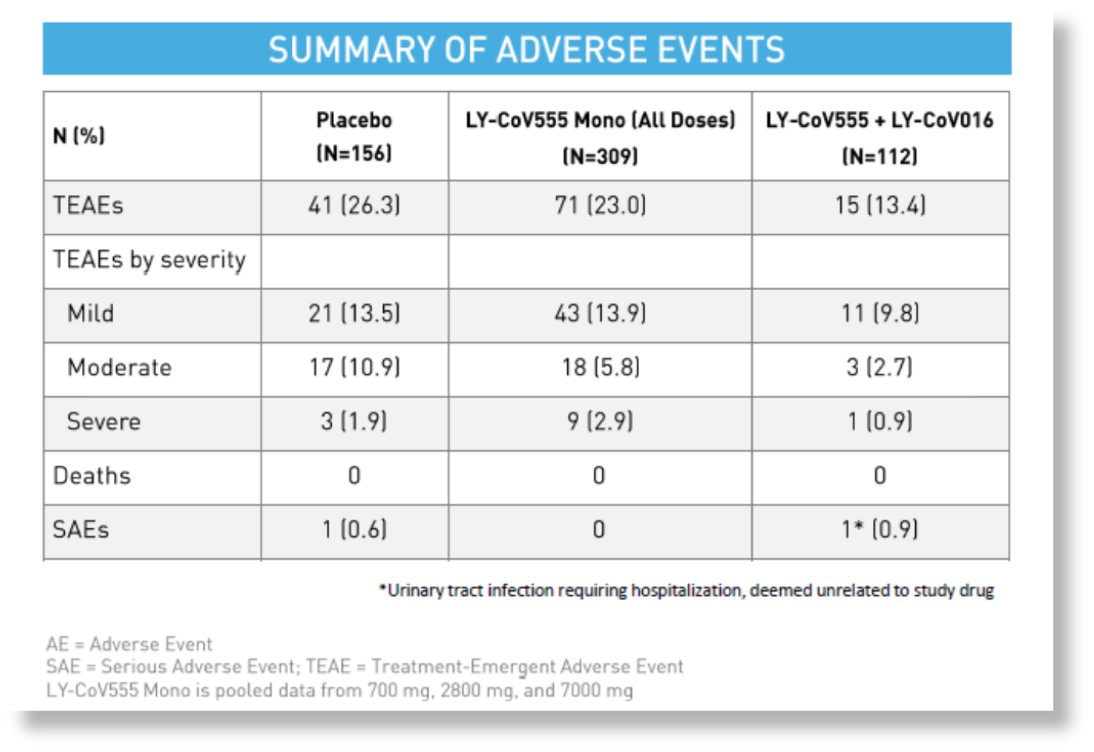
*data from Eli Lilly
Combination therapy appears more efficacious on viral endpoints
- Primary endpoint of change from baseline in viral load at Day 11 was met
- Secondary endpoints of reduced viral load at Day 3 and Day 7, as well as time-weighted average change from baseline for Day 1 to Day 11 were met
- Exploratory analyses show a reduction in the 1% of patients with persistently high viral load at Day 7
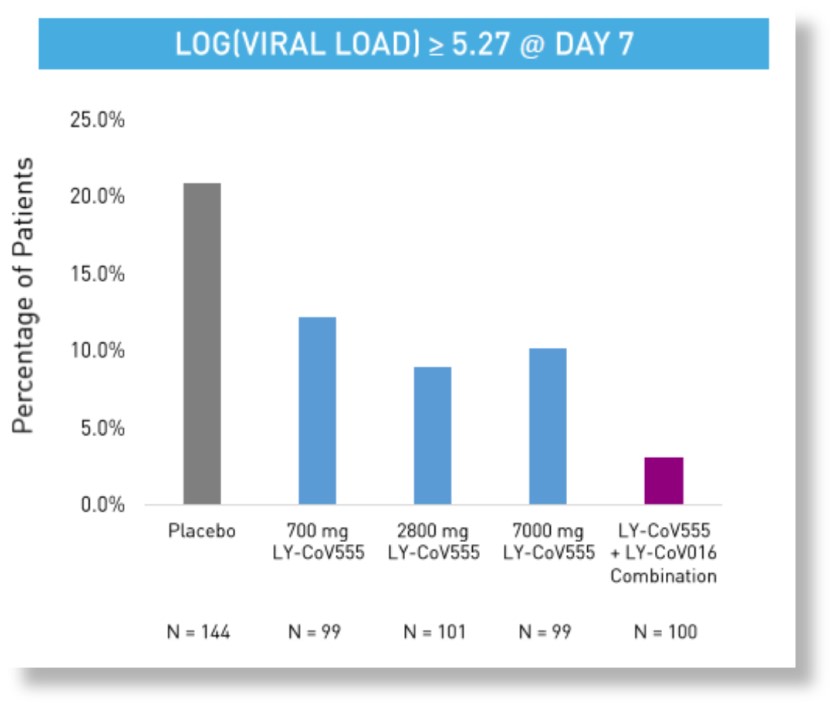
*data from Eli Lilly
Scientific recognition by peer reviewed journals
- Specifically Recognize & Blocks the Binding of RBD
- First Neutralizing Antibody Trial in Non-human Primates
- Demonstrated potent SARS-CoV-2-specific neutralization activity in vitro against SARS-CoV-2
- Lowered the risk of Fc-mediated acute lung injury

